

No serious adverse or thrombotic events were reported. Andexanet alfa treatment also reduced unbound, pharmacologically active concentrations of both FXa inhibitors and restored TF-initiated TG (TF-TG) compared with placebo. The reversal effect was sustained throughout the infusion and for approximately 2 hours following the end of infusion compared with placebo. When administered as a bolus or a bolus plus 2-hour infusion in subjects receiving anticoagulation for 4 days, andexanet alfa immediately and significantly reversed apixaban or rivaroxaban-associated anti-FXa activity.
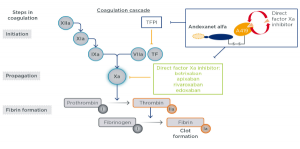
In Part 1 of the studies, subjects received andexanet alfa as a 400 mg (ANNEXA-A) or 800 mg (ANNEXA-R) bolus, whereas subjects in Part 2 received the same bolus plus a 480 mg (4 mg/min, ANNEXA-A) or 960 mg (8 mg/min, ANNEXA-R) infusion of andexanet alfa. The Phase III ANNEXA-A (apixaban) and ANNEXA-R (rivaroxaban) studies evaluated the effects of andexanet alfa versus placebo on healthy, older volunteers treated with 5 mg of apixaban twice daily or 20 mg of rivaroxaban daily, respectively. ANDEXANET ALFA REVERSES THE ANTICOAGULATION EFFECTS OF APIXABAN AND RIVAROXABAN


 0 kommentar(er)
0 kommentar(er)
